Representing mixtures with State objects
The State object provides a full picture of the themochemical state of a gaseous
mixture. It can be used to extract missing thermochemical quantities, or to keep a
meaningful thermochemical state while modifying individual state variables. A State
does not contain any velocity or numerical information.
State initialization
A State object can be initialized in two ways:
From the temperature, pressure, and species mass fractions \((T, P, Y_k)\) through the default constructor:
state = State(T, P, Yk)
From conservative variables \((\rho, \rho E, \rho Y_k)\) through the
from_consconstructor:state = State.from_cons(rho, rhoE, rhoYk)
\(E\) is the total energy defined as the sum of the sensible and chemical (formation) energies ([TNC] p. 3)
The constructor arguments \((T, P, Y_k)\) or \((\rho, \rho E, \rho Y_k)\) can be scalars or multidimensional arrays.
Warning
When initializing from conservative variables, \(T\) is determined by a Newton-Raphson method to ensure that the mixture energy matches the input energy. This is an expensive step that may take a long time for large inputs.
The following flowchart details how from_cons works
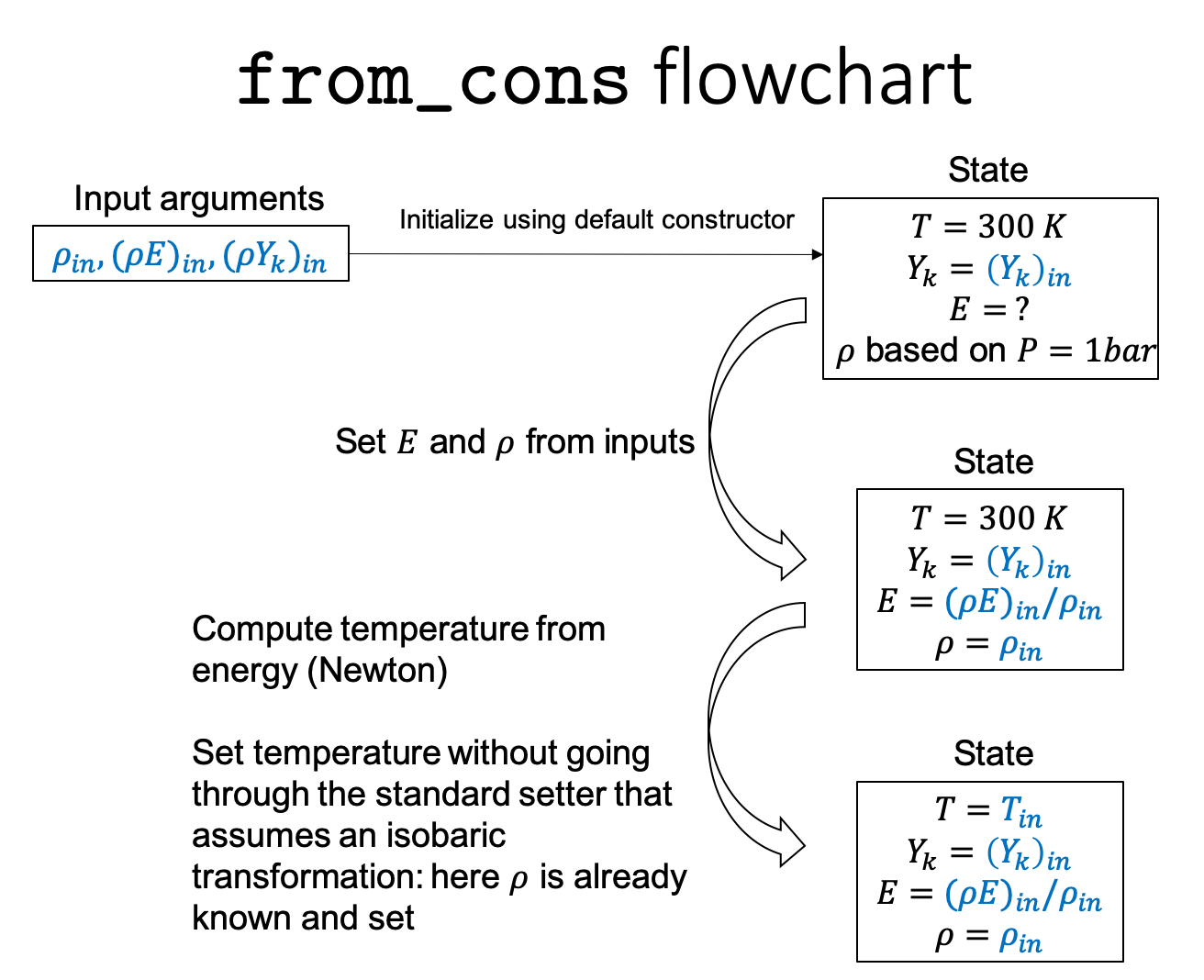
State transformations
After a State has been initialized, \(T\), \(P\) and \(Y_k\) can
independently be set to new values (e.g. myState.temperature = newTemperature) and the other state variables are modified
accordingly:
When setting a new value for \(T\), the other state variables are modified assuming an isobaric and iso-composition transformation from the previous state.
When setting a new value for \(P\), the other state variables are modified assuming an isothermal and iso-composition transformation from the previous state.
When setting a new value for \(Y_k\), the other state variables are modified assuming an isothermal and isobaric transformation from the previous state.
State transformations always satisfy the perfect gas equation of state
- TNC
Poinsot, T.; Veynante, D. Theoretical and Numerical Combustion, 3rd ed.; 2011.